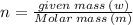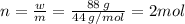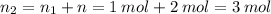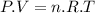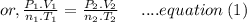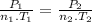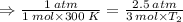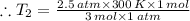Chemistry, 28.12.2019 04:31 hilljade45
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.0 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.5 atm. what is the final temperature? assume the solid carbon dioxide takes up negligible volume.
a. 150 k
b. 200 k
c. 250 k
d. 300 k
e. 400 k

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide....
Questions



History, 22.06.2019 05:50

History, 22.06.2019 05:50





Mathematics, 22.06.2019 05:50

Business, 22.06.2019 05:50

Mathematics, 22.06.2019 05:50

Mathematics, 22.06.2019 05:50


English, 22.06.2019 05:50

History, 22.06.2019 05:50




Mathematics, 22.06.2019 05:50