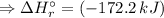Chemistry, 28.12.2019 05:31 lisamiller
Calculate horxn for the following reaction: h3aso4(aq) + 4 h2(g) --> ash3(g) + 4 h2o(l)(hof [ash3(g)] = 66.4 kj/mol; hof [h3aso4(aq)] = -904.6 kj/mol; hof [h2o(l)] = -285.8 kj/mol)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Calculate horxn for the following reaction: h3aso4(aq) + 4 h2(g) --> ash3(g) + 4 h2o(l)(hof [a...
Questions



Arts, 20.11.2021 14:00

Biology, 20.11.2021 14:00

Arts, 20.11.2021 14:00

Health, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00



Chemistry, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00



Chemistry, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00



Mathematics, 20.11.2021 14:00


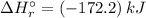
![\Delta H_{f}^{\circ } [H_{3}AsO_{4}(aq)]](/tpl/images/0435/4880/bc889.png) = -904.6 kJ/mol
= -904.6 kJ/mol![\Delta H_{f}^{\circ } [H_{2}(g)]](/tpl/images/0435/4880/b83d7.png) = 0 kJ/mol,
= 0 kJ/mol,![\Delta H_{f}^{\circ } [AsH_{3}(g)]](/tpl/images/0435/4880/eaaf4.png) = +66.4 kJ/mol
= +66.4 kJ/mol![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/4880/46f61.png) = -285.8 kJ/mol
= -285.8 kJ/mol 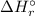 = ?
= ?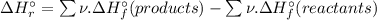
![\Delta H_{r}^{\circ } = [1 \times \Delta H_{f}^{\circ } [AsH_{3} (g)] + 4 \times \Delta H_{f}^{\circ } [H_{2}O(l)]] - [1 \times \Delta H_{f}^{\circ } [H_{3}AsO_{4}(aq)] + 4 \times \Delta H_{f}^{\circ } [H_{2}(g)]](/tpl/images/0435/4880/cee8f.png)
![\Rightarrow \Delta H_{r}^{\circ } = [1 \times (+66.4\,kJ/mol) + 4 \times (-285.8\,kJ/mol) ] - [1 \times (-904.6\,kJ/mol) + 4 \times (0\,kJ/mol)]](/tpl/images/0435/4880/49bdc.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-1076.8\, kJ] - [-904.6\,kJ]](/tpl/images/0435/4880/a7183.png)