Chemistry, 28.12.2019 05:31 viktoria1198zz
At a particular temperature the first-order gas-phase reaction n 2o 5→ 2no 2 + (1/2)o 2 has a half-life for the disappearance of dinitrogen pentoxide of 5130 s. suppose 0.450 atm of n 2o 5 is introducted into an evacuated 2.00 l flask. what will be the total gas pressure inside the flask after 3.00 hours? a. 0.969 atm b. 0.105 atm c. 0.795 atm d. 1.14 atm e. 0.864 atm

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
At a particular temperature the first-order gas-phase reaction n 2o 5→ 2no 2 + (1/2)o 2 has a half-l...
Questions

Social Studies, 02.02.2021 01:20


Mathematics, 02.02.2021 01:20




Mathematics, 02.02.2021 01:20




Mathematics, 02.02.2021 01:20

Mathematics, 02.02.2021 01:20


World Languages, 02.02.2021 01:20


Mathematics, 02.02.2021 01:20

Law, 02.02.2021 01:20


Mathematics, 02.02.2021 01:20

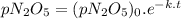
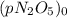 is the initial pressure
is the initial pressure