
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) 2 hi (g), are [h2] = 0.106 m; [i2] = 0.022 m; [hi] = 1.29 m calculate the new equilibrium concentration of hi (in m) if the equilibrium concentrations of h2 and i2 are 0.95 m and 0.019 m respectively.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) ...
Questions

Chemistry, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40



Mathematics, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40


Mathematics, 10.11.2020 20:40

Mathematics, 10.11.2020 20:40

Health, 10.11.2020 20:40




Advanced Placement (AP), 10.11.2020 20:40

History, 10.11.2020 20:40




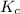 ) for the given chemical reaction, is given by the equation:
) for the given chemical reaction, is given by the equation:![K_{c} = \frac {[HI]^{2}}{[H_{2}]\: [I_{2}]}](/tpl/images/0435/6174/f8601.png)
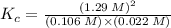
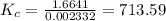
![K_{c} = \frac {[HI]^{2}}{(0.95\: M) \times (0.019\: M)}](/tpl/images/0435/6174/d2511.png)
![\Rightarrow K_{c} = 713.59 = \frac {[HI]^{2}}{0.01805}](/tpl/images/0435/6174/fef3c.png)
![\Rightarrow [HI]^{2} = 713.59 \times 0.01805 = 12.88](/tpl/images/0435/6174/d4ab1.png)
![\Rightarrow [HI] = \sqrt {12.88} = 3.589 M](/tpl/images/0435/6174/5120a.png)


