
Astudent is doing a titration using potassium permanganate solution, kmno4, to determine the amount of h2o2 in a sample. the balanced equation for the reaction in the titration is given below:
2 mno4-(aq) + 6 h+(aq) + 5 h2o2(aq) --> ž 2 mn2+(aq) + 8 h2o(l) + 5 o2(g)
a student calculates an amount of moles of h2o2 that is larger than the actual value. which of the following errors could correctly explain the larger value?
the student failed to wear goggles.
the student did not swirl the flask appropriately and therefore stopped short of the endpoint.
the student failed to rinse the buret with kmno4¬ solution after rinsing it with distilled water.
the student added an extra 15 ml of distilled water to the h2o2 solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
Astudent is doing a titration using potassium permanganate solution, kmno4, to determine the amount...
Questions


Mathematics, 08.09.2020 23:01

Mathematics, 08.09.2020 23:01



Mathematics, 08.09.2020 23:01

Mathematics, 08.09.2020 23:01





Mathematics, 08.09.2020 23:01




English, 08.09.2020 23:01

Mathematics, 08.09.2020 23:01

Biology, 08.09.2020 23:01


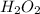 solution.
solution.

