
Chemistry, 31.12.2019 00:31 niescarlosj
The combustion of ammonia by the following reaction yields nitric oxide and water
4 nh3 (g) + 5 o2 (g) → 4 no (g) + 6 h2o (g)
determine the heat of reaction (δhrxn) for this reaction by using the following thermochemical data:
n2 (g) + o2 (g) → 2 no (g) δh = 180.6 kj
n2 (g) + 3 h2 (g) → 2 nh3 (g) δh = - 91.8 kj
2 h2 (g) + o2 (g) → 2 h2o (g) δh = - 483.7 kj
hess' law problems
2. determine the heat of reaction (δhrxn) for the process by which hydrazine (n2h4) is formed from its elements:
n2 (g) + 2 h2 (g) → n2h4 (g)
by using the following thermochemical data:
n2h4 (g) + o2 (g) → n2 (g) + 2 h2o (g) δh = - 622.2 kj
h2 (g) + 1/2 o2 (g) → h2o (g) δh = - 285.8 kj
hess' law problems
3. determine the heat of reaction (δhrxn) for the process by which lead (ii) chloride (pbcl2) is formed from its elements:
pb (s) + cl2 (g) → pbcl2 (s)
by using the following thermochemical data:
pb (s) + 2 cl2 (g) → pbcl4 (l) δh = - 329.3 kj
pbcl2 (s) + cl2 (g) → pbcl4 (l) δh = + 30.1 kj
hess' law problems
4. determine the heat of reaction (δhrxn) for the process by which acetic acid (ch3cooh) is formed from its elements:
c (graphite) + 2 h2 (g) + o2 (g) → ch3cooh (l)
by using the following thermochemical data:
ch3cooh (l) + o2 → 2 co2 (g) + 2 h2o(l) δh = - 871 kj
c (graphite) + o2 (g) → 2 co2 (g) δh = - 394 kj
h2 (s) + 1/2 o2 (g) → h2o(l) δh = - 286 kj
heat of formation problems
5. determine the heat of reaction (δhrxn) for the combustion of ethanol (c2h5oh) by using heat of formation data:
c2h5oh (l) + 3 o2 (g) → 2 co2 + 3 h2o (g)
heat of formation problems
6. determine the heat of reaction (δhrxn) for the chlorination of methane (ch4) by using heat of formation data:
ch4 (g) + 4 cl2 (g) → ccl4 (g) + 4 hcl (g)
heat of formation problems
7. determine the heat of reaction (δhrxn) for the reduction of acetaldehyde (ch3cho) to ethanol (c2h5oh) by using heat of formation data:
ch3cho (g) + h2 (g) → c2h5oh (l)
heat of formation problems
8. determine the heat of reaction (δhrxn) for the reaction of calcium carbonate (caco3) with hcl to produce co2 by using heat of formation data:
caco3 (s) + 2 hcl (g) → cacl2 (s) + co2 (g) + h2o (g)
expert answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

You know the right answer?
The combustion of ammonia by the following reaction yields nitric oxide and water
4 nh3...
4 nh3...
Questions


Mathematics, 17.03.2020 19:56

Mathematics, 17.03.2020 19:56

History, 17.03.2020 19:56


Mathematics, 17.03.2020 19:56

Mathematics, 17.03.2020 19:56

English, 17.03.2020 19:56






Mathematics, 17.03.2020 19:56


Mathematics, 17.03.2020 19:56


Mathematics, 17.03.2020 19:56


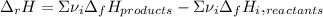
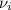 accounts for the stoichiometric coefficient of the ith compound based on the standard enthalpies of formation.
accounts for the stoichiometric coefficient of the ith compound based on the standard enthalpies of formation.




