
Chemistry, 31.12.2019 01:31 icantspeakengles
Asolution of cacl 2 in water forms a mixture that is 42.0 % calcium chloride by mass. if the total mass of the mixture is 839.6 g, what masses of cacl 2 and water were used

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Asolution of cacl 2 in water forms a mixture that is 42.0 % calcium chloride by mass. if the total m...
Questions

Biology, 08.03.2021 22:50



Mathematics, 08.03.2021 22:50

Mathematics, 08.03.2021 22:50


Social Studies, 08.03.2021 22:50


Biology, 08.03.2021 22:50

Mathematics, 08.03.2021 22:50

Mathematics, 08.03.2021 22:50

English, 08.03.2021 22:50

Mathematics, 08.03.2021 22:50

Mathematics, 08.03.2021 22:50

Biology, 08.03.2021 22:50


Chemistry, 08.03.2021 22:50

Chemistry, 08.03.2021 22:50

Mathematics, 08.03.2021 22:50

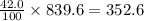 grams of calcium chloride
grams of calcium chloride

