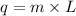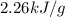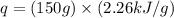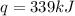Answers: 3


Another question on Chemistry



Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
Show the calculation of the energy involved in condensation of 150 grams of steam at 100oc if the he...
Questions

Advanced Placement (AP), 20.12.2019 00:31

History, 20.12.2019 00:31

Mathematics, 20.12.2019 00:31

Mathematics, 20.12.2019 00:31

History, 20.12.2019 00:31



Mathematics, 20.12.2019 00:31


Mathematics, 20.12.2019 00:31


Mathematics, 20.12.2019 00:31




Chemistry, 20.12.2019 00:31

Biology, 20.12.2019 00:31


Social Studies, 20.12.2019 00:31