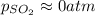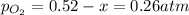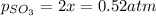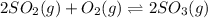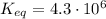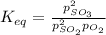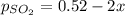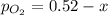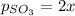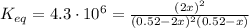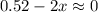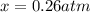Chemistry, 31.12.2019 01:31 aaliyahettorre
2so2(g) + o2(g) equilibrium reaction arrow 2 so3(g) calculate the equilibrium partial pressures of so2, o2, and so3 produced from an initial mixture in which the partial pressures of so2 and o2 = 0.52 atm and the partial pressure of so3 = 0 (exactly).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
2so2(g) + o2(g) equilibrium reaction arrow 2 so3(g) calculate the equilibrium partial pressures of s...
Questions


History, 14.11.2019 23:31

Physics, 14.11.2019 23:31

History, 14.11.2019 23:31







Biology, 14.11.2019 23:31


Biology, 14.11.2019 23:31





Mathematics, 14.11.2019 23:31

History, 14.11.2019 23:31

English, 14.11.2019 23:31