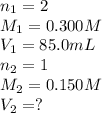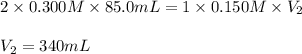Chemistry, 31.12.2019 02:31 htorres2p8urw0
How many milliliters of 0.150 m naoh are required to neutralize 85.0 ml of 0.300 m h2so4 ? the balanced neutralization reaction is: h2so4(aq)+2naoh(aq)→na2so4(aq)+2h2o (l).

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

You know the right answer?
How many milliliters of 0.150 m naoh are required to neutralize 85.0 ml of 0.300 m h2so4 ? the bala...
Questions

Mathematics, 19.11.2020 20:30

Mathematics, 19.11.2020 20:30

Mathematics, 19.11.2020 20:30



English, 19.11.2020 20:30

Mathematics, 19.11.2020 20:30


Arts, 19.11.2020 20:30

History, 19.11.2020 20:30



Mathematics, 19.11.2020 20:30

Mathematics, 19.11.2020 20:30

Mathematics, 19.11.2020 20:30



English, 19.11.2020 20:30


 required to neutralize is, 340 mL
required to neutralize is, 340 mL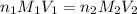
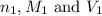 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 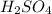
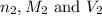 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.