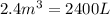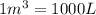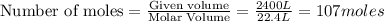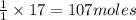Chemistry, 31.12.2019 03:31 kalcius674
Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. what volume of carbon dioxide would be produced by this reaction if of carbon disulfide were consumed? also, be sure your answer has a unit symbol, and is rounded to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Carbon disulfide gas and oxygen gas react to form sulfur dioxide gas and carbon dioxide gas. what vo...
Questions





Chemistry, 16.10.2019 18:20

Chemistry, 16.10.2019 18:20














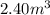
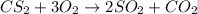
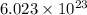 of particles.
of particles.