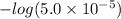Chemistry, 31.12.2019 04:31 James300102
Calculate the ph of a buffer solution prepared by dissolving 0.20 mole of cyanic acid (hcno) and 0.80 mole of sodium cyanate (nacno) in enough water to make 1.0 liter of solution. [k a(hcno) = 2.0 × 10 −4] 0.97 3.10 4.40 3.70 4.30

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Calculate the ph of a buffer solution prepared by dissolving 0.20 mole of cyanic acid (hcno) and 0.8...
Questions

Mathematics, 05.11.2021 22:20




History, 05.11.2021 22:20


Mathematics, 05.11.2021 22:30

Mathematics, 05.11.2021 22:30

History, 05.11.2021 22:30



Chemistry, 05.11.2021 22:30


Mathematics, 05.11.2021 22:30

English, 05.11.2021 22:30





Mathematics, 05.11.2021 22:30

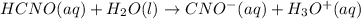
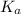 of HCNO is
of HCNO is 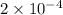 mol.
mol.![K_{a} = \frac{[CNO^{-}][H_{3}O^{+}]}{[HCNO]}](/tpl/images/0437/9975/6a3d8.png)
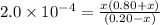
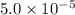 M
M ![-log[H_{3}O^{+}]](/tpl/images/0437/9975/74ef2.png)