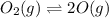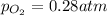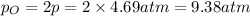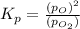Chemistry, 31.12.2019 04:31 angelespinosa521
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure of 4.97 atm, where it decomposes to o(g) by the reaction below.
o2(g) ⇄ 2 o(g)
at equilibrium, the partial pressure of o2 is 0.28 atm. calculate kp for this reaction at 4224 k.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure o...
Questions







Spanish, 19.03.2021 20:40

Mathematics, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40




History, 19.03.2021 20:40

English, 19.03.2021 20:40

Chemistry, 19.03.2021 20:40


Mathematics, 19.03.2021 20:40

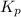 at 4224 K is 314.23.
at 4224 K is 314.23.