

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
Aprecipitation reaction is caused by mixing 100. ml of 0.25 m k2cr2o7 solution with 100. ml of 0.25...
Questions


Mathematics, 14.09.2019 08:30


Mathematics, 14.09.2019 08:30




Computers and Technology, 14.09.2019 08:30




Computers and Technology, 14.09.2019 08:30

Computers and Technology, 14.09.2019 08:30



Computers and Technology, 14.09.2019 08:30


Computers and Technology, 14.09.2019 08:30

Mathematics, 14.09.2019 08:30

Computers and Technology, 14.09.2019 08:30

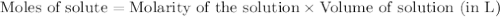 .....(1)
.....(1)
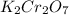 solution = 0.25 M
solution = 0.25 M
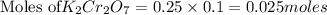
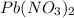 solution = 0.25 M
solution = 0.25 M
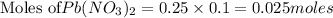
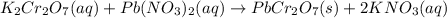
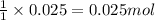 of lead dichromate
of lead dichromate


