

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
1. the specific heat capacity of iron is 0.461 j g–1 k–1 and that of titanium is 0.544 j g–1 k–1. a...
Questions

Spanish, 12.02.2021 06:20

Spanish, 12.02.2021 06:20

Mathematics, 12.02.2021 06:20



Mathematics, 12.02.2021 06:20

Mathematics, 12.02.2021 06:20

Mathematics, 12.02.2021 06:20


Mathematics, 12.02.2021 06:20

Biology, 12.02.2021 06:20

History, 12.02.2021 06:20




English, 12.02.2021 06:20



History, 12.02.2021 06:20

Computers and Technology, 12.02.2021 06:20

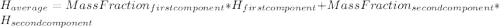
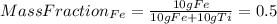
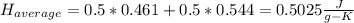
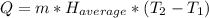
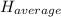 : The value obtained in equation (3)
: The value obtained in equation (3)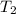 : Final temperature of the sample
: Final temperature of the sample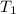 : Initial temperature of the sample
: Initial temperature of the sample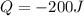 The mass of the whole sample is: 10g of Fe + 10g of Ti = 20g of sampleThe temperatures must be in absolute units of temperature (these are: rankine or kelvin)The initial temperature of the system is 100°C or 373K
The mass of the whole sample is: 10g of Fe + 10g of Ti = 20g of sampleThe temperatures must be in absolute units of temperature (these are: rankine or kelvin)The initial temperature of the system is 100°C or 373K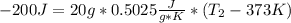
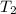
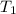 )
)

