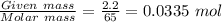Chemistry, 31.12.2019 05:31 carlosleblanc26
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many liters of hydrogen gas are produced by placing a zinc nail with a mass of 2.2g into an excess of hydrochloric acid.
a) 0.0015 l b) 0.75 l c) 1.33 l d) 6.42 l

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
You know the right answer?
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many...
at conditions of standard temperature and pressure, determine how many...
Questions


History, 05.05.2020 11:28

Chemistry, 05.05.2020 11:28

Mathematics, 05.05.2020 11:28


Mathematics, 05.05.2020 11:28



History, 05.05.2020 11:28



Mathematics, 05.05.2020 11:28

Chemistry, 05.05.2020 11:28

Mathematics, 05.05.2020 11:28

Business, 05.05.2020 11:28


Mathematics, 05.05.2020 11:28

Mathematics, 05.05.2020 11:28

Mathematics, 05.05.2020 11:28

Mathematics, 05.05.2020 11:28