

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Calculate the lattice energy of kcl(s) given the following data using the born-haber cycle: δhsubli...
Questions





Business, 13.07.2019 06:50


Social Studies, 13.07.2019 06:50



Social Studies, 13.07.2019 06:50

History, 13.07.2019 06:50

Biology, 13.07.2019 06:50

Biology, 13.07.2019 06:50

Biology, 13.07.2019 06:50

Biology, 13.07.2019 06:50


History, 13.07.2019 06:50


Social Studies, 13.07.2019 06:50

Biology, 13.07.2019 06:50

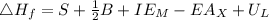
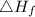 is enthalpy of formation
is enthalpy of formation 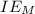 is ionisation enthalpy of metal
is ionisation enthalpy of metal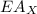 is electron affinity of non metal atom
is electron affinity of non metal atom 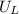 is lattice energy
is lattice energy 

