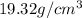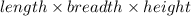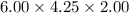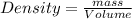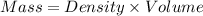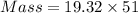Chemistry, 02.01.2020 04:31 jonellelewis2897
What is the difference in mass between 3.01×10^24 atoms of gold and a gold bar with the dimensions 6.00 cm x 4.25 cm x 2.00 cm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
What is the difference in mass between 3.01×10^24 atoms of gold and a gold bar with the dimensions 6...
Questions

Mathematics, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00





Computers and Technology, 21.04.2021 07:00

Chemistry, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00

Business, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00




Chemistry, 21.04.2021 07:00

Spanish, 21.04.2021 07:00


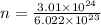
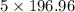 g
g atoms of gold = 984.5 g
atoms of gold = 984.5 g