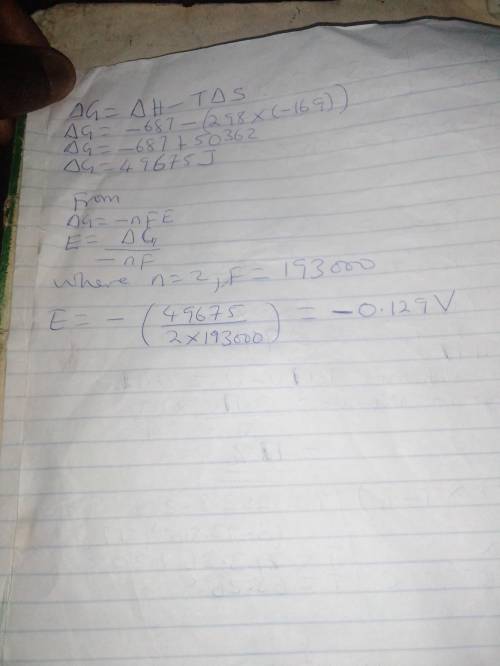Chemistry, 02.01.2020 22:31 Kiaraboyd9366
Calculate the standard cell potential at 25 ∘c for the reaction x(s)+2y+(aq)→x2+(aq)+2y(s) where δh∘ = -687 kj and δs∘ = -169 j/k .
express your answer to three significant figures and include the appropriate

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1


Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
Calculate the standard cell potential at 25 ∘c for the reaction x(s)+2y+(aq)→x2+(aq)+2y(s) where δh∘...
Questions


Physics, 06.10.2019 08:50

English, 06.10.2019 08:50

Mathematics, 06.10.2019 08:50


History, 06.10.2019 08:50



Physics, 06.10.2019 08:50

Physics, 06.10.2019 08:50



Mathematics, 06.10.2019 08:50

Social Studies, 06.10.2019 08:50


History, 06.10.2019 08:50

English, 06.10.2019 08:50

Biology, 06.10.2019 08:50