
Chemistry, 02.01.2020 23:31 kyleemarie2003
Which substance in each of the following pairs would you expect to have the higher boiling point? explain why.
a. ne or xe
b. co2 or cs2
c. ch4 or cl2
d. f2 or lif
e. nh3 or ph3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
Which substance in each of the following pairs would you expect to have the higher boiling point? e...
Questions

Geography, 27.07.2019 01:30

Geography, 27.07.2019 01:30

Geography, 27.07.2019 01:30


Geography, 27.07.2019 01:30






English, 27.07.2019 01:30

Computers and Technology, 27.07.2019 01:30

Mathematics, 27.07.2019 01:30



Mathematics, 27.07.2019 01:30




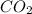 and
and 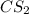 are non-polar in nature with intermolecular dispersion forces. So, more is the molecular weight of a compound more heat will be required by it in order to break the bonds.
are non-polar in nature with intermolecular dispersion forces. So, more is the molecular weight of a compound more heat will be required by it in order to break the bonds. 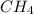 is 16 g/mol and molecular weight of
is 16 g/mol and molecular weight of 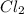 is 35 g/mol.
is 35 g/mol. 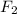 is a covalent compound so, it will also be non-polar in nature. Hence, it has weak intermolecular dispersion forces. On the other hand, LiF is an ionic compound and it has strong intermolecular forces of attraction due to the presence of opposite charges on the combining atoms.
is a covalent compound so, it will also be non-polar in nature. Hence, it has weak intermolecular dispersion forces. On the other hand, LiF is an ionic compound and it has strong intermolecular forces of attraction due to the presence of opposite charges on the combining atoms. 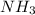 and
and 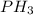 are polar molecules in which dipole-dipole forces does exist. Since, nitrogen atom is more electronegative than phosphorous atom so, it will have stronger hydrogen bonding and strong intermolecular forces.
are polar molecules in which dipole-dipole forces does exist. Since, nitrogen atom is more electronegative than phosphorous atom so, it will have stronger hydrogen bonding and strong intermolecular forces.

