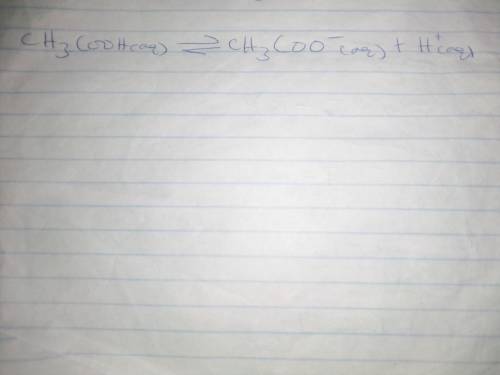Chemistry, 03.01.2020 02:31 Isaiahtate053
Which of the following factors would be most likely to cause acetic acid to completely dissociate in aqueous solution?
a. higher temperatures, which increase the pka of the acid
b. enzymes that catalyze the forward reaction
c. continuous addition of acetic acid to the solution
d. continuous removal of protons from the solution

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Which of the following factors would be most likely to cause acetic acid to completely dissociate in...
Questions

Biology, 19.11.2020 21:20

English, 19.11.2020 21:20






Mathematics, 19.11.2020 21:20

English, 19.11.2020 21:20


History, 19.11.2020 21:20

German, 19.11.2020 21:20

History, 19.11.2020 21:20

History, 19.11.2020 21:20


Mathematics, 19.11.2020 21:20


English, 19.11.2020 21:20