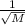Choose a depiction of a gas sample containing equal molar amounts of xenon and argon as described by kinetic molecular theory. red dots are used to represent xenon atoms and blue dots to represent argon atoms. each atom is drawn with a "tail" that represents its velocity relative to the others in the mixture.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Choose a depiction of a gas sample containing equal molar amounts of xenon and argon as described by...
Questions



Mathematics, 03.04.2020 19:39


Spanish, 03.04.2020 19:40


Social Studies, 03.04.2020 19:40




Mathematics, 03.04.2020 19:41





Mathematics, 03.04.2020 19:41

Biology, 03.04.2020 19:41



Mathematics, 03.04.2020 19:41

 ∝
∝