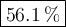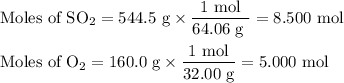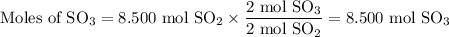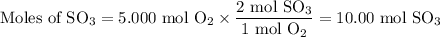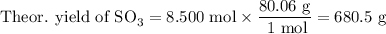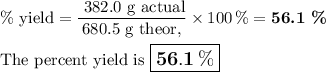Chemistry, 05.01.2020 19:31 johnandashley5p65r4a
What is the percent yield of the reaction below when 544.5 g so2 and 160.0g o2 produce 382.0 g so3? 2so2+o2+2so3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
What is the percent yield of the reaction below when 544.5 g so2 and 160.0g o2 produce 382.0 g so3?...
Questions


Mathematics, 18.01.2021 20:00

Engineering, 18.01.2021 20:00

Mathematics, 18.01.2021 20:00


Chemistry, 18.01.2021 20:00

Arts, 18.01.2021 20:00

Computers and Technology, 18.01.2021 20:00


Social Studies, 18.01.2021 20:00

Mathematics, 18.01.2021 20:00

Mathematics, 18.01.2021 20:10

Mathematics, 18.01.2021 20:10

Computers and Technology, 18.01.2021 20:10

French, 18.01.2021 20:10

Mathematics, 18.01.2021 20:10



Mathematics, 18.01.2021 20:10