
Chemistry, 06.01.2020 04:31 Silkyruthie
Consider the half reactions below for a chemical reaction.
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e⁻> cu(s)
what is the overall equation for this chemical reaction?
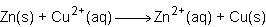

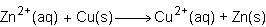
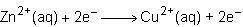

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
Consider the half reactions below for a chemical reaction.
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e...
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e...
Questions




Mathematics, 18.03.2021 02:20

Social Studies, 18.03.2021 02:20

Arts, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20

Biology, 18.03.2021 02:20





Chemistry, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20








