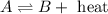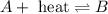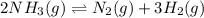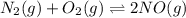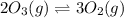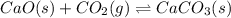Chemistry, 06.01.2020 19:31 karsynraine9419
How will an increase in temperature affect each of the following equilibria? how will a decrease in the volume of the reaction vessel affect each?
(a) 2nh3(g) ⇌ n2(g)+3h2(g) δh = 92kj
(b) n2(g) + o2(g) ⇌ 2no(g) δh =181kj
(c) 2o3(g) ⇌ 3o2(g) δh = − 285kj
(d) cao(s) + co2(g) ⇌ caco3(s) δh = − 176kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
How will an increase in temperature affect each of the following equilibria? how will a decrease in...
Questions

History, 31.01.2020 08:48

Biology, 31.01.2020 08:48

Mathematics, 31.01.2020 08:48



English, 31.01.2020 08:48







English, 31.01.2020 08:49

Mathematics, 31.01.2020 08:49

Physics, 31.01.2020 08:49

Mathematics, 31.01.2020 08:49


Mathematics, 31.01.2020 08:49


Mathematics, 31.01.2020 08:49