

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Which one of the following solutes is most likely to have low water solubility due to the dissolutio...
Questions

Mathematics, 20.09.2019 07:30

Chemistry, 20.09.2019 07:30

Mathematics, 20.09.2019 07:30

Mathematics, 20.09.2019 07:30




Social Studies, 20.09.2019 07:30


Social Studies, 20.09.2019 07:30

Biology, 20.09.2019 07:30


Mathematics, 20.09.2019 07:30


Chemistry, 20.09.2019 07:30

History, 20.09.2019 07:30

Mathematics, 20.09.2019 07:30



English, 20.09.2019 07:30

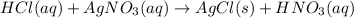
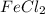 and
and 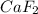 are ionic compound and these are highly soluble in water.
are ionic compound and these are highly soluble in water.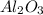 is slightly soluble in water as it is a covalent compound.
is slightly soluble in water as it is a covalent compound.

