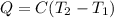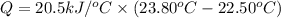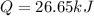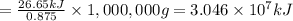A0.875-g sample of anthracite coal was burned in a bomb calorimeter. the temperature rose from 22.50 to 23.80°c. the heat capacity of the calorimeter was found in another experiment to be 20.5 kj/°c.
a. what was the heat evolved by the reaction?
b. what is the energy released on burning 1 metric ton (exactly 1000 kg) of this type of coal?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2
You know the right answer?
A0.875-g sample of anthracite coal was burned in a bomb calorimeter. the temperature rose from 22.50...
Questions

History, 02.11.2019 16:31

Mathematics, 02.11.2019 16:31



Physics, 02.11.2019 16:31



Mathematics, 02.11.2019 16:31

Mathematics, 02.11.2019 16:31


Mathematics, 02.11.2019 16:31


Biology, 02.11.2019 16:31



Biology, 02.11.2019 16:31


Health, 02.11.2019 16:31


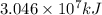 is the energy released on burning 1 metric ton of this type of coal
is the energy released on burning 1 metric ton of this type of coal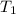 = 22.50°C
= 22.50°C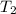 = 23.80°C
= 23.80°C