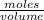Chemistry, 08.01.2020 02:31 bernicewhite156
Suppose you are a food chemist working for a company that makes and manufactures soda. your job is to create a new soft drink with the ingredients available to you. there is one problem—you need 1 liter of phosphoric acid solution, and the phosphoric acid available to use has a concentration of 0.01 m. this is much too high to add to your soda without distorting the flavor. typically, there are about 45 milligrams of phosphoric acid per 12 ounce can of soda. here is your task:
convert the 45 milligrams of phosphoric acid (h3po4) into grams and then into moles.
0.00046 moles or 4.6×10-4 moles
given that 12 ounces is equal to about 0.35 liter, find the molarity of the phosphoric acid in a typical can of soda (45 milligrams). 0.0013 m or 1.3×10-3 m
using the dilution equation, determine how much of the stock solution (0.01 m h3po4) you will need to make 1 liter of the concentration typically used in soda.
0.13 liter or 130 milliliters

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 08:30
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2
You know the right answer?
Suppose you are a food chemist working for a company that makes and manufactures soda. your job is t...
Questions


History, 06.09.2019 01:20

History, 06.09.2019 01:20

Mathematics, 06.09.2019 01:20




Mathematics, 06.09.2019 01:20


English, 06.09.2019 01:20



English, 06.09.2019 01:20





Mathematics, 06.09.2019 01:30

Mathematics, 06.09.2019 01:30