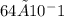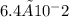Chemistry, 08.01.2020 06:31 monkeys450
What mass of cdp (403 g mol−1) is in 10 ml of the buffered solution at the beginning of experiment 1? 6.4 × 10−4 g 6.4 × 10−3 g 6.4 × 10−2 g 6.4 × 10−1 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
What mass of cdp (403 g mol−1) is in 10 ml of the buffered solution at the beginning of experiment 1...
Questions

Mathematics, 30.05.2020 01:59

Biology, 30.05.2020 01:59

Physics, 30.05.2020 01:59


English, 30.05.2020 01:59

Health, 30.05.2020 01:59


Mathematics, 30.05.2020 01:59


History, 30.05.2020 01:59



History, 30.05.2020 01:59






Biology, 30.05.2020 01:59

History, 30.05.2020 01:59

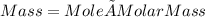
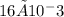 moles of CDP in 1 liter of buffer.
moles of CDP in 1 liter of buffer.