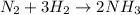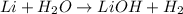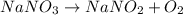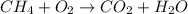2. classify each of the following reactions as a
synthesis, decomposition, single-displacement,
double-displacement, or combustion reaction:
a. n.(g) + 3h2(g) +2nh2(g)
b. 2li(s) + 2h,0(1) -2lioh(aq) + h2(g)
c. 2nano3(s) +2nano3(s) + 0,(9)
d. 2ch() + 190,(g) 1200,(g) + 14h, o(1)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
2. classify each of the following reactions as a
synthesis, decomposition, single-displacement,...
synthesis, decomposition, single-displacement,...
Questions

Mathematics, 10.02.2021 22:50




Social Studies, 10.02.2021 22:50

Chemistry, 10.02.2021 22:50

English, 10.02.2021 22:50





Mathematics, 10.02.2021 22:50

History, 10.02.2021 22:50


Mathematics, 10.02.2021 22:50


Mathematics, 10.02.2021 22:50