
Chemistry, 09.01.2020 01:31 zachspencer6444
Given the following information, what is the concentration of h2o(g) at equilibrium? [h2s](eq) = 0.671 m [o2](eq) = 0.587 m kc = 1.35 2h2s(g) + o2(g) ⇌ 2s(s) + 2h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Given the following information, what is the concentration of h2o(g) at equilibrium? [h2s](eq) = 0....
Questions



Chemistry, 12.02.2022 06:00


Biology, 12.02.2022 06:00

English, 12.02.2022 06:00


Mathematics, 12.02.2022 06:00

Computers and Technology, 12.02.2022 06:00

Engineering, 12.02.2022 06:00





History, 12.02.2022 06:00



Health, 12.02.2022 06:00

Mathematics, 12.02.2022 06:00

History, 12.02.2022 06:00

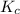
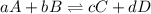
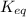 is written as:
is written as:![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0447/6972/b6f47.png)
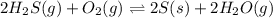
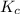 for above equation is:
for above equation is:![K_c=\frac{[H_2O]^2}{[H_2S]^2\times [O_2]}](/tpl/images/0447/6972/73c62.png)
![[H_2S]_{eq}=0.671M](/tpl/images/0447/6972/623cf.png)
![[O_2]_{eq}=0.587M](/tpl/images/0447/6972/4ff7f.png)

![1.35=\frac{[H_2O]^2}{(0.671)^2\times 0.587}](/tpl/images/0447/6972/14791.png)
![[H_2O]=\sqrt{(1.35\times 0.671\times 0.671\times 0.587)}=0.597M](/tpl/images/0447/6972/73db2.png)


