
Chemistry, 09.01.2020 01:31 chloedonyes
For the reaction, 2 n2o5--> 4 no2+o2, the rate of formation of no2 is 0.004 mol^-1 s^-1.a) calculate the rate of disappearance of n2o5.b) calculate the rate of appearance of o2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
For the reaction, 2 n2o5--> 4 no2+o2, the rate of formation of no2 is 0.004 mol^-1 s^-1.a) calcu...
Questions



Mathematics, 05.01.2021 22:40



Spanish, 05.01.2021 22:40


Mathematics, 05.01.2021 22:40




Mathematics, 05.01.2021 22:40

Chemistry, 05.01.2021 22:40

Social Studies, 05.01.2021 22:40

Mathematics, 05.01.2021 22:40


Health, 05.01.2021 22:40

Biology, 05.01.2021 22:40

English, 05.01.2021 22:40

English, 05.01.2021 22:40

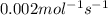
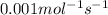
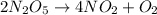
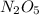 =
= ![-\frac{1d[N_2O_5]}{2dt}](/tpl/images/0447/6971/30fba.png)
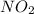 =
= ![\frac{1d[NO_2]}{4dt}](/tpl/images/0447/6971/b576c.png)
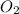 =
= ![\frac{1d[O_2]}{dt}](/tpl/images/0447/6971/6b167.png)
![NO_2=+\frac{1d[NO_2]}{dt}=0.004mol^{-1}s^{-1}](/tpl/images/0447/6971/9cf34.png)
![N_2O_5=\frac{2}{4}\times \frac{1d[NO_2]}{dt}=\frac{2}{4}\times 0.004=0.002mol^{-1}s^{-1}](/tpl/images/0447/6971/202ca.png)
![\frac{1}{4}\times \frac{1d[NO_2]}{dt}=\frac{1}{4}\times 0.004=0.001mol^{-1}s^{-1}](/tpl/images/0447/6971/a8812.png)


