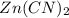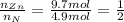Chemistry, 09.01.2020 03:31 twalters88
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number. measurements also show that a certain sample of the unknown compound contains 9.7 mol of nitrogen and 4.9 mol of zinc. write the complete chemical formula for the unknown compound.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number....
Questions







English, 28.02.2020 04:54

Computers and Technology, 28.02.2020 05:15

Mathematics, 28.02.2020 05:15

Physics, 28.02.2020 05:15


English, 28.02.2020 05:15


Mathematics, 28.02.2020 05:15


Mathematics, 28.02.2020 05:15

Mathematics, 28.02.2020 05:15