
Chemistry, 09.01.2020 06:31 loanyst99111
If the vapor pressure of ethanol at 34.7degree c is 100
mmhg, and its heat of vaporization is 38.6 kj/mol. at what
temperaturewould ethanol boil at sea level?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
If the vapor pressure of ethanol at 34.7degree c is 100
mmhg, and its heat of vaporization is...
mmhg, and its heat of vaporization is...
Questions


Mathematics, 05.05.2020 01:23

Mathematics, 05.05.2020 01:23

English, 05.05.2020 01:23





Biology, 05.05.2020 01:23

Mathematics, 05.05.2020 01:23



English, 05.05.2020 01:23

English, 05.05.2020 01:23

Mathematics, 05.05.2020 01:23

Computers and Technology, 05.05.2020 01:23

History, 05.05.2020 01:23


Mathematics, 05.05.2020 01:23

![\frac{ΔvapH}{R}([tex]\frac{1}{T1}](/tpl/images/0448/1509/1cff8.png) -
-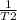 )
)

