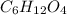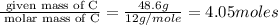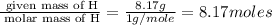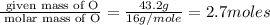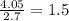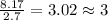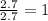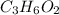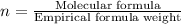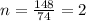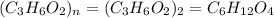Chemistry, 09.01.2020 06:31 alleshia2007
Kethoxal, a substance used to combat viruses, is 48.6% carbon, 8.17% hydrogen, and 43.2% oxygen. it has a molar mass of 148 g / mol. what is the molecular formula of kethoxal?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
Kethoxal, a substance used to combat viruses, is 48.6% carbon, 8.17% hydrogen, and 43.2% oxygen. it...
Questions

Biology, 03.06.2021 07:40

Physics, 03.06.2021 07:40

Mathematics, 03.06.2021 07:40

Mathematics, 03.06.2021 07:40




Mathematics, 03.06.2021 07:40

Mathematics, 03.06.2021 07:40


Mathematics, 03.06.2021 07:40

Mathematics, 03.06.2021 07:40

Mathematics, 03.06.2021 07:40



Mathematics, 03.06.2021 07:40

Mathematics, 03.06.2021 07:40

Computers and Technology, 03.06.2021 07:40

Mathematics, 03.06.2021 07:50

Mathematics, 03.06.2021 07:50