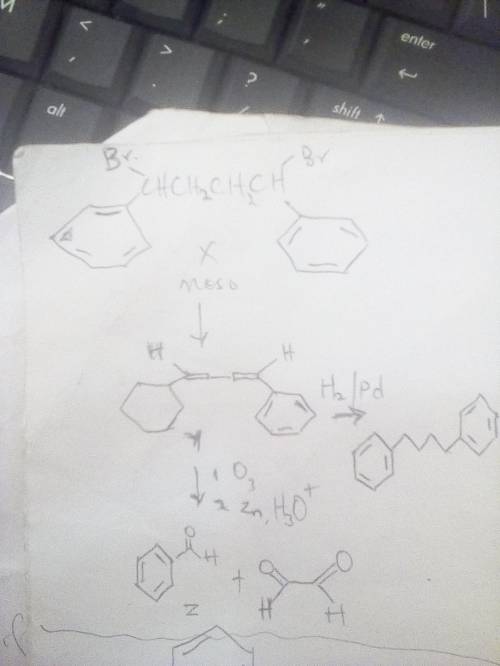
Compound x is optically inactive and has the
formulac16h16br2. on treatment
withstrong b...

Chemistry, 09.01.2020 06:31 kelseiroll8554
Compound x is optically inactive and has the
formulac16h16br2. on treatment
withstrong base, x gives hydrocarbon y,
c16h14.compound y absorbs 2 equivalents of
hydrogen when reduced over apalladium catalyst and reacts with
ozone to give two fragments. onefragment, z, is an aldehyde with
formulac7h6o. the other fragment is
glyoxal,(cho)2. write the reactions involved, and
suggeststructures for x, y, and z. what is the stereochemistry of
x?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
Questions

Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

Biology, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

History, 09.01.2021 01:00

Arts, 09.01.2021 01:00






History, 09.01.2021 01:00

Physics, 09.01.2021 01:00

History, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00