Consider a solution containing 0.181 m lead ions and 0.365
miron(ii) ions. the ksp for lead su...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Questions


English, 30.05.2021 16:50


Mathematics, 30.05.2021 16:50


Geography, 30.05.2021 16:50

Geography, 30.05.2021 16:50

Mathematics, 30.05.2021 17:00


Mathematics, 30.05.2021 17:00



Mathematics, 30.05.2021 17:00




English, 30.05.2021 17:00

Mathematics, 30.05.2021 17:00

Computers and Technology, 30.05.2021 17:00

Geography, 30.05.2021 17:00

![[H_3O^+]=6.8*10^{-22}](/tpl/images/0448/0948/8039f.png)
![[Pb^{+2}]=1*10^{-6}](/tpl/images/0448/0948/21ac4.png) :
:![Kps=[Pb^{+2}]*[S^{-2}]=3.4*10^{-28}](/tpl/images/0448/0948/0133d.png)
![1*10^{-6}*[S^{-2}]=3.4*10^{-28}](/tpl/images/0448/0948/5bca8.png)
![[S^{-2}]=3.4*10^{-22}](/tpl/images/0448/0948/c45b5.png)
![Kps=[0.365]*[S^{-2}]=3.7*10^{-19}](/tpl/images/0448/0948/c04d1.png)
![[S^{-2}]=1.01*10^{-18}](/tpl/images/0448/0948/5c3d9.png)
![[S^{-2}]](/tpl/images/0448/0948/d1351.png) concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate.
concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate. 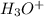
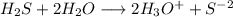
![[H_3O^+]=2*[S^{-2}]=6.8*10^{-22}](/tpl/images/0448/0948/3de14.png)


