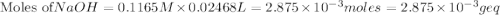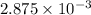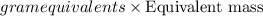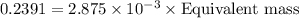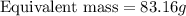Chemistry, 09.01.2020 06:31 roseemariehunter12
If is found that 24.68 ml of .1165 m naoh is needed to titrate .2931 g of an unknown acid to the phenolphthalein end point. calculate the equivlanet mass of the acid.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2

Chemistry, 23.06.2019 10:00
Which element forms a compound with chlorine with the general formula mci?
Answers: 1

Chemistry, 23.06.2019 15:40
Sugar is made up of clear, colorless crystals that dissolve easily in water, but the crystals and their solution do not conduct electricity. which statement describes sugar? it is made up of atoms that are held together by metallic bonds. it is made up of atoms that are held together by covalent bonds, it is made up of atoms that are held together by weak ionic bonds. it is made up of atoms that are held together by strong ionic bonds.
Answers: 1
You know the right answer?
If is found that 24.68 ml of .1165 m naoh is needed to titrate .2931 g of an unknown acid to the phe...
Questions

History, 19.01.2021 23:10

Social Studies, 19.01.2021 23:10


Mathematics, 19.01.2021 23:10

History, 19.01.2021 23:10


Chemistry, 19.01.2021 23:10

Mathematics, 19.01.2021 23:10


Mathematics, 19.01.2021 23:10


Mathematics, 19.01.2021 23:10



Biology, 19.01.2021 23:10


History, 19.01.2021 23:10


Health, 19.01.2021 23:10

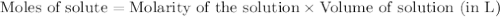
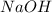 solution = 0.1165 M
solution = 0.1165 M