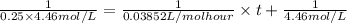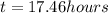At 25 deg. c, the second-order reaction nocl(g)no(g)+
1/2 cl2(g) is 50% complete after 5.82 ho...

Chemistry, 09.01.2020 07:31 genyjoannerubiera
At 25 deg. c, the second-order reaction nocl(g)no(g)+
1/2 cl2(g) is 50% complete after 5.82 hours when the
initialconcentration of nocl is 4.46 mol/l. how long will it
takefor the reaction to be 75% complete?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

You know the right answer?
Questions

History, 30.03.2020 20:25

Mathematics, 30.03.2020 20:25







Mathematics, 30.03.2020 20:25




History, 30.03.2020 20:25

Chemistry, 30.03.2020 20:25

History, 30.03.2020 20:25




Mathematics, 30.03.2020 20:25

Mathematics, 30.03.2020 20:25

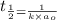
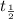 = half life = 5.82 hour
= half life = 5.82 hour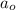 = initial concentration = 4.46 mol/L
= initial concentration = 4.46 mol/L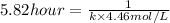
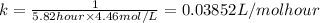
![\frac{1}{[a]}=kt+\frac{1}{[a_o]}](/tpl/images/0448/2354/dfc33.png)
![100\%-75\%=25\% of [a_o]=0.25[a_o]](/tpl/images/0448/2354/5eba6.png)