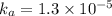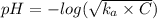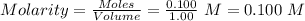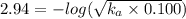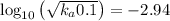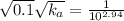Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3
You know the right answer?
Asolution prepared by dissolving 0.100 mole of propionic acid in enough water to make 1.00 l of solu...
Questions




Biology, 23.04.2021 05:10






Mathematics, 23.04.2021 05:10

Computers and Technology, 23.04.2021 05:10


Chemistry, 23.04.2021 05:10




Mathematics, 23.04.2021 05:10

Mathematics, 23.04.2021 05:10

English, 23.04.2021 05:10