Urgent plese and you
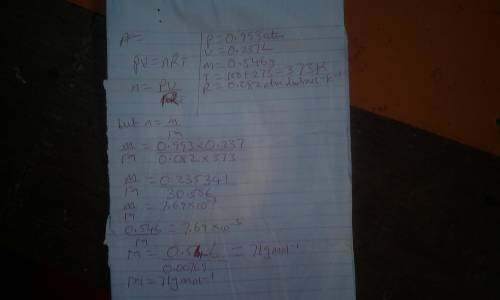
an ideal gas sample weighing .546 g at 100 degrees celsius and .993 atm...

Chemistry, 11.01.2020 03:31 monsterduckgoose
Urgent plese and you
an ideal gas sample weighing .546 g at 100 degrees celsius and .993 atm has a volume of .237 l. determine the molar mass of the gas.
a. 71.3 g/mol
b. 143 g/mol
c. 19.1 g/mol
d. 0140 g/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
Questions



Mathematics, 02.02.2021 06:40

Biology, 02.02.2021 06:40

History, 02.02.2021 06:40

Mathematics, 02.02.2021 06:40

Advanced Placement (AP), 02.02.2021 06:40

Mathematics, 02.02.2021 06:40

Mathematics, 02.02.2021 06:40




Mathematics, 02.02.2021 06:40

Mathematics, 02.02.2021 06:40





English, 02.02.2021 06:40