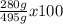Chemistry, 12.01.2020 02:31 jakobrobinette
What is the % yield when 140.0 grams of ethylene gas (c2h4) reacts with excess chlorine to form 280.0 grams of 1,2-dichloro ethane (c2h4cl2)? (given equation is c2h4 + cl2 —> c2h4cl2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
What is the % yield when 140.0 grams of ethylene gas (c2h4) reacts with excess chlorine to form 280....
Questions



English, 02.07.2019 19:00

Mathematics, 02.07.2019 19:00

Social Studies, 02.07.2019 19:00

Mathematics, 02.07.2019 19:00

Mathematics, 02.07.2019 19:00


History, 02.07.2019 19:00

Mathematics, 02.07.2019 19:00


Advanced Placement (AP), 02.07.2019 19:00

Spanish, 02.07.2019 19:00


Social Studies, 02.07.2019 19:00


English, 02.07.2019 19:00

Mathematics, 02.07.2019 19:00

Mathematics, 02.07.2019 19:00