
In an experiment, potassium chlorate decomposed according to the following chemical equation. kclo3 → kcl + o2 (molar mass of kclo3 = 122.5 g/mol; kcl = 74.55 g/mol; o2 = 31.998 g/mol) if the mass of kcl produced was 180 grams, which of the following calculations can be used to determine the mass of potassium chlorate decomposed? (180 × 2 × 74.55) ÷ (122.5 × 2) grams (180 × 3 × 74.55) ÷ (122.5 × 2) grams (180 × 2 × 122.5) ÷ (74.55 × 2) grams (180 × 3 × 122.5) ÷ (74.55 × 2) grams

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Infants born with severe respiratory problems are sometimes given liquid ventilation: they breathe a liquid that can dissolve more oxygen than air can hold. one of these liquids is a fluorinated compound, cf3(cf2)7br. the solubility of oxygen in this liquid is 66 mlo2 per 100 ml liquid. in contrast, air is 21 % oxygen by volume. calculate the moles of o2 present in an infant's lungs (volume: 12 ml ) if the infant takes a full breath of air. assume a pressure of 1 atm in the lungs.
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2
You know the right answer?
In an experiment, potassium chlorate decomposed according to the following chemical equation. kclo3...
Questions





Health, 04.11.2019 02:31

History, 04.11.2019 02:31


History, 04.11.2019 02:31


Biology, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31

History, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31

History, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31

Spanish, 04.11.2019 02:31


Mathematics, 04.11.2019 02:31

Mathematics, 04.11.2019 02:31

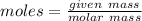
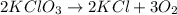
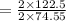 of KClO3
of KClO3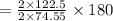 of KClO3
of KClO3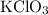 has been given by
has been given by 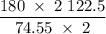 grams. Thus option C is correct.
grams. Thus option C is correct.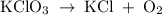
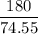 moles
moles molecular weight
molecular weight

