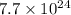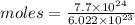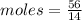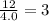Chemistry, 13.01.2020 09:31 leopolesamoy
Aparticular compound in the chemistry laboratory is found to contain 7.2x10^24 atoms of oxygen, 56.0g of nitrogen, and 4.0 mol of hydrogen. what is it’s empirical formula?


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Aparticular compound in the chemistry laboratory is found to contain 7.2x10^24 atoms of oxygen, 56.0...
Questions

Mathematics, 24.03.2021 17:40


Chemistry, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Computers and Technology, 24.03.2021 17:40







Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40





Arts, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40

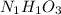
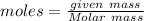
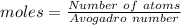
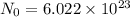 atoms
atoms