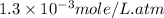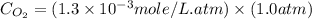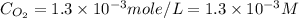Chemistry, 13.01.2020 21:31 aneisha0117
What is the molar concentration of oxygen in water at 25°c for a partial pressure of 1.0 atm ( = 1.3 x 10⁻³ mol/latm) a. 7.2 x 10⁻⁴b. 1.3 x 10⁻³ m c. 7.7 x 10⁴d. 1.3 x 10⁻⁵
= 1.3 x 10⁻³ mol/latm) a. 7.2 x 10⁻⁴b. 1.3 x 10⁻³ m c. 7.7 x 10⁴d. 1.3 x 10⁻⁵

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
What is the molar concentration of oxygen in water at 25°c for a partial pressure of 1.0 atm ([tex]k...
Questions

Mathematics, 17.12.2020 01:10


English, 17.12.2020 01:10

Advanced Placement (AP), 17.12.2020 01:10

Biology, 17.12.2020 01:10

Biology, 17.12.2020 01:10


Mathematics, 17.12.2020 01:10

Biology, 17.12.2020 01:10




Mathematics, 17.12.2020 01:10

Mathematics, 17.12.2020 01:10

History, 17.12.2020 01:10


Mathematics, 17.12.2020 01:10

Advanced Placement (AP), 17.12.2020 01:10

Mathematics, 17.12.2020 01:10

History, 17.12.2020 01:10

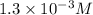
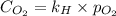
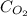 = molar concentration of
= molar concentration of 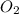 = ?
= ?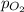 = partial pressure of
= partial pressure of 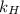 = Henry's law constant =
= Henry's law constant =