rate = k[n_2]^3 [o_3]

Chemistry, 14.01.2020 00:31 helphelp81
The rate of a certain reaction is given by the following rate law:
rate = k[n_2]^3 [o_3]
use this information to answer the questions below.
1. what is the reaction order in n_2?
2. what is the reaction order in o_3?
3. what is overall reaction order?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
The rate of a certain reaction is given by the following rate law:
rate = k[n_2]^3 [o_3]
rate = k[n_2]^3 [o_3]
Questions

Computers and Technology, 30.01.2020 00:44

Health, 30.01.2020 00:44


History, 30.01.2020 00:44


History, 30.01.2020 00:44

English, 30.01.2020 00:44


Social Studies, 30.01.2020 00:44

History, 30.01.2020 00:44


Mathematics, 30.01.2020 00:44

Social Studies, 30.01.2020 00:44


Health, 30.01.2020 00:45


History, 30.01.2020 00:45

Mathematics, 30.01.2020 00:45

Mathematics, 30.01.2020 00:45

Mathematics, 30.01.2020 00:45

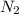 = 3
= 3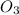 = 1
= 1![rate = k[N_2]^3 [O_3]](/tpl/images/0453/5258/fc831.png)


