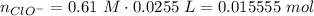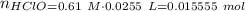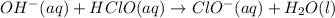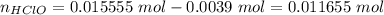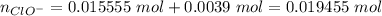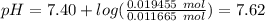Abuffer solution with a volume of 0.0255 l consists of 0.61 m hypochlorous acid (hclo), a weak acid, plus 0.61 m potassium hypochlorite (kclo). the acid dissociation constant of hypochlorous acid, ka, is 4.0 x 10^−8.
1. determine the ph of the buffer solution after the addition of 0.0039 mol rubidium hydroxide (rboh), a strong base. (assume no change in solution volume.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Abuffer solution with a volume of 0.0255 l consists of 0.61 m hypochlorous acid (hclo), a weak acid,...
Questions







Biology, 24.06.2019 09:50

Biology, 24.06.2019 09:50

Biology, 24.06.2019 09:50

Biology, 24.06.2019 09:50

Biology, 24.06.2019 09:50

Physics, 24.06.2019 09:50

Mathematics, 24.06.2019 09:50




Mathematics, 24.06.2019 09:50


English, 24.06.2019 09:50

![pH = pK_a + log(\frac{[A^-]}{[HA]})](/tpl/images/0453/4960/e07d5.png)
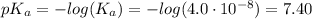
![[A^-] = [ClO^-]](/tpl/images/0453/4960/01383.png)
![[HA] = [HClO]](/tpl/images/0453/4960/a69d6.png)