
Most wine is prepared by the fermentation of the glucose in grape juice by yeast: c6h12o6(aq) --> 2c2h5oh(aq) + 2co2(g) how many grams of glucose should there be in grape juice to produce 725 mls of wine that is 11.0% ethyl alcohol, c2h5oh (d=0.789 g/cm3), by volume?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Most wine is prepared by the fermentation of the glucose in grape juice by yeast: c6h12o6(aq) -->...
Questions

History, 01.11.2019 11:31


Chemistry, 01.11.2019 11:31

English, 01.11.2019 11:31


Mathematics, 01.11.2019 11:31





Mathematics, 01.11.2019 11:31


History, 01.11.2019 11:31

Biology, 01.11.2019 11:31



Chemistry, 01.11.2019 11:31

Mathematics, 01.11.2019 11:31

Biology, 01.11.2019 11:31

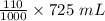 = 79.75 mL
= 79.75 mL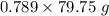 = 62.92 g
= 62.92 g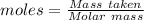
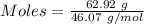
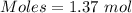
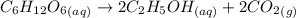
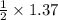 mole of glucose reacts
mole of glucose reacts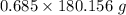 = 123.41 g
= 123.41 g 

