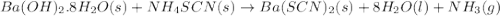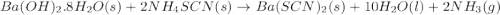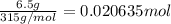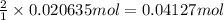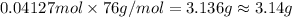Chemistry, 14.01.2020 06:31 brookemcelhaney
One of relatively few reactions that takes place directly between two solids at room temperature is in this equation, the in ba(oh)2 indicates the presence of eight water molecules. this compound is called barium hydroxide octahydrate.
a. balance the equation.
b. what mass of ammonium thiocyanate (nh4scn) must be used if it is to react completely with 6.5 g barium hydroxide octahydrate?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

You know the right answer?
One of relatively few reactions that takes place directly between two solids at room temperature is...
Questions



Chemistry, 23.09.2019 03:30

Computers and Technology, 23.09.2019 03:30

Social Studies, 23.09.2019 03:30




Mathematics, 23.09.2019 03:30

Mathematics, 23.09.2019 03:30

Mathematics, 23.09.2019 03:30

History, 23.09.2019 03:30


Chemistry, 23.09.2019 03:30

Physics, 23.09.2019 03:40





World Languages, 23.09.2019 03:50