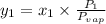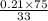Chemistry, 14.01.2020 20:31 danielamejia13
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assuming that benzene and toluene from and idea solution.
a) what is the composition in mole fractions of a solution that has a vapor pressure of 33 torr at 20 °c
b) what is the mole fraction of benzene in the vapor above the solution described in part a?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1
You know the right answer?
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assu...
Questions

Mathematics, 15.06.2021 15:50


Mathematics, 15.06.2021 15:50



Mathematics, 15.06.2021 15:50

English, 15.06.2021 15:50


Mathematics, 15.06.2021 15:50


Mathematics, 15.06.2021 15:50

History, 15.06.2021 15:50

Mathematics, 15.06.2021 15:50

History, 15.06.2021 15:50

History, 15.06.2021 15:50



Mathematics, 15.06.2021 15:50

Mathematics, 15.06.2021 15:50

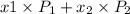
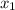 = mole fraction of component one
= mole fraction of component one
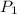 = vapor pressure of component one when pure
= vapor pressure of component one when pure
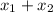 = 1
= 1
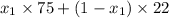
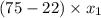
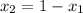
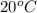 is 0.79.
is 0.79.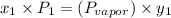
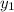 = composition in gas phase
= composition in gas phase